More about SARS-COV2 vaccines
There is a lack of evidence that SARS-COV2 vaccines protect against the disease. One observational trial involving PLWH found lower levels of IgG SARS-COV2 antibodies than those of non-HIV participants. However, these results are limited because PLWH has a high prevalence of comorbidities, which increase the risk of infection by the SARS-CoV2 virus. Furthermore, PLWH without ART have low CD4 cell counts and are expected to have poorer responses to treatment for SARS-CoV2.
Conavidecia is another SARS-COV2-based vaccine, which relies on a recombinant adenovirus type-5 (Ad5) vector to express the SARS-CoV2 surface spike protein. It was developed by two biotechnology companies, Beijing Institute of Biotech and CanSino Biological, and was initially tested on a group of 508 volunteers. The first dose of this vaccine contains a recombinant adenoviral vector (rAd5 vector) that expresses the full-length SARS-CoV2 spike protein.
Although the efficacy of the current SARS-COV2 vaccines is still at around 90%, their protective efficacy has decreased over time due to the emergence of new virus variants. However, this does not mean that the vaccines are ineffective in preventing the spread of SARS-CoV2. More research needs to be done to define the correlates of protection with each vaccine and establish a central database. In addition, booster doses may improve immunogenicity, and others may develop alternative vaccines to provide adequate protection.
ChAdOx1 nCoV-19 vaccine is another SARS-COV2 vaccine. This vaccine was developed by Oxford University and AstraZeneca and comprised a replication-deficient simian adenovirus vector that encodes the SARS CoV-2 spike protein. In this first preliminary Phase I/II trial, the nCoV-19 vaccine was administered to 1077 healthy adults aged 18 to 55. The vaccine was also given to 10 volunteers in a non-randomized prime-boost group.
The two SARS-COV2 vaccines currently on the market are very effective in preventing the onset of symptoms. The latest data from the PHE shows that the COVID-19 vaccine is effective against the original SARS-CoV2 virus and its variants. In addition to its efficacy against the original SARS CoV2, the vaccine has shown high protection against Delta and Beta variants, although the data on these variants is limited.
The Ad5-nCoV vaccine induced a strong immune response and increased neutralizing antibodies in most participants. This vaccine has since been advanced to Phase III clinical trials. However, further studies are required to determine if it can induce a Th2 response. In addition, there is a lack of evidence on immune responses’ Th1/Th2 polarization.
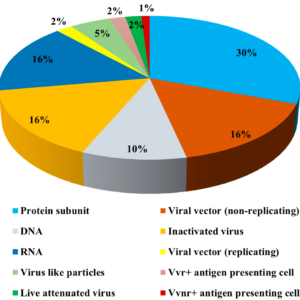
Several vaccine candidates are being developed to combat the SARS-CoV2 virus. Some of them are currently in phase I and II clinical trials. However, many of them should be ready for clinical trials within the next 14 months. The goal is to develop a vaccine that confers at least 50% protection against COVID-19 infection.
There are currently four mRNA-based vaccine candidates for SARS-COV-2. The most promising of these is the BNT162 vaccine, developed in collaboration with BioNTech. This vaccine consists of nucleotide-modified RNA that encodes the full-length SARS CoV-2 spike protein antigen.
In phase one of the trial, the vaccine was given to 131 healthy subjects with a placebo. 27% of participants experienced a fever. The second dose had a significantly lower incidence of fever. A higher proportion of participants reported fatigue and headache than the first dose. The most common gastrointestinal side effect was nausea. Interestingly, only 7.7% of the participants reported nausea after the second dose.
Nevertheless, the response to the SARS-CoV2 vaccine was excellent in pwMS. Although immunosuppressive DMTs may reduce the response, they should offer SARS-CoV2 vaccinations to all MS patients. They should tailor the timing of vaccination according to the a-priori risk and DMT status.
A highly effective vaccine is the best route to return to “normalcy.”
Alternative measures include widespread testing of the population, rigorous trace-back of contacts, and readily available therapeutics. Furthermore, a more effective vaccine may reduce severe illness and death cases, leading to lower healthcare costs and the spread of the virus.
A Phase I clinical trial conducted in the UK and Brazil showed that a single dose of the Ad5-nCoV2 vaccine effectively against the SARS-COV2 virus. It produced significant neutralizing antibodies at days 14 and 28 and induced specific T-cell responses.
However, some participants suffered from a severe infections. Some participants also experienced joint and muscle pain.